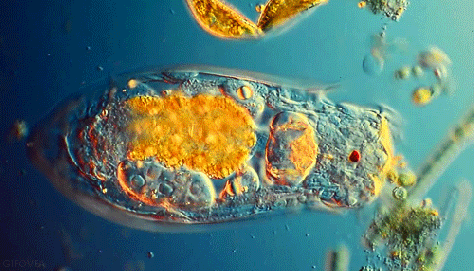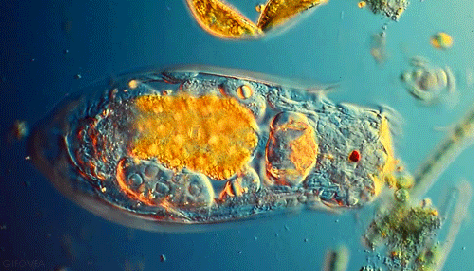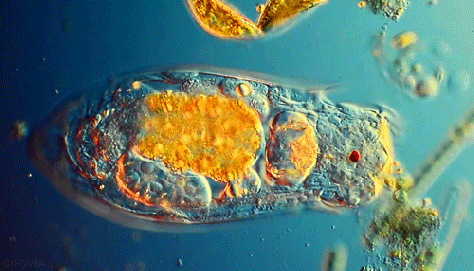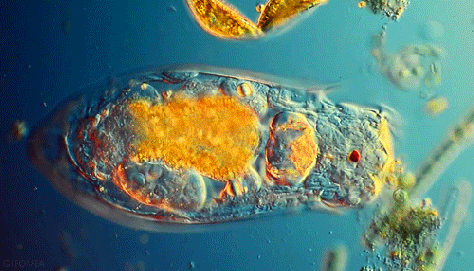
Microbes are beautiful. I am not much of an expert on protozoa but I’ve seen a lot of these little guys under the microscope in soil samples. Protozoa are single celled but eukaryotic microbes, which basically means they are distinguished from other single celled microbes by their complex internal structure and generally larger size. Protozoa are an important indicator of soil fertility and, by consuming smaller bacteria and excreting nutrients, play an important role in delivering nutrients to plant roots in a complex process known as the microbial loop.
Tag Archives: microbe
The history beneath your feet
I think about dirt a lot more than most people. Probably this is the result of my background in ecosystem science, the study of how nutrients and energy flow throughout the biosphere. The soil represents a huge sink for all major nutrients that sustain life on earth, and an important source of nutrients such as carbon and nitrogen that are naturally recycled to the atmosphere.
But it’s not economic, or really even environmental value that makes soil so fascinating to me. It’s history. In the forests of northeastern Puerto Rico where I’m currently conducting research, the historic record deposited in soil is up to 8, 10 or 15 meters thick and stretches back 300,000 years into the past, to a time when the island itself was shooting up out of the Gulf of Mexico due to rising magmatic rock deep beneath the ocean floor.
If you start looking closely at the composition of soil, you will quickly discover a wealth of information recorded within it. Tiny grains of minerals, produced from thousands to millions of years of water dissolving rock, form the physical matrix on which life has developed. As these clean, crystalline minerals slowly rot, they are chemically transformed into new compounds such as clays. Clays and other secondary minerals add stickiness to the soil, allowing decomposing organic materials to adhere. Slowly, a strange collection of organic materials that were once living and the inorganic ingredients that supported their existence begins to accumulate. As this assortment of the dead and rotting grows, so does the living biomass that it sustains. Most of the soil microfauna is involved in feeding off dead (or other living) organic materials and ultimately recycling nutrients that would otherwise be locked away forever. Embracing death is a way of life in the world’s most biologically diverse ecosystem.
But wait- I was talking about history. Yes, living organisms, dead organic materials, clays and minerals are all important components of the soil, but how do we piece together a history (and of what? The geology that the soil formed over? The forest that once stood atop it? The long-dead animals whose traces still linger within it?) from such a complex and dynamic system?
The answer is not entirely clear. But I am convinced that history is sitting in the dirt, rotting away, waiting patiently for someone to find a way to unwrap the stories contained within.
Quorum sensing: an understudied control in plant nutrient availability
It is a well known fact that many bacteria like to live in groups. Moreover, just as human societies strive to facilitate group living, (through grocery stores that provide food for otherwise unsustainably crowded cities, and municipal waste centers to ensure that our concentrated living spaces are kept clean enough to remain livable), bacterial populations employ a variety of strategies that enhance collective life.
The bacterial analog to human group-living strategies is called quorum sensing. Quorum sensing, or QS, is really an umbrella term used to describe a range of bacterial behaviors that enhance group life. QS behaviors are considered common to all bacteria and have likely been tracking bacterial evolution since the first true cells of Precambrian earth crawled out of their prebiotic soup. Here I’ll focus on a particular group of bacteria that employ some very ecologically important QS strategies.
Bacteria are broadly divided into several distinct phyla that occupy a range of earth habitats and employ an enormous variety of survival strategies. Proteobacteria are one such major group that is particularly interesting to me because of their posited dominance in the zone of plant-nutrient uptake known as the rhizosphere. The significance of proteobacteria in rhizosphere microbial populations has really only been examined in temperate forests, and hopefully some of my own work will bring a tropical perspective to our understanding of rhizosphere community composition.
It turns out that proteobacteria release a specific signal molecule known as N-acyl-homoserine lactone, or (AHL) in order to alert other proteobacteria of their existence. Thus proteobacteria are able to “quorum sense” their environment and receive information of the relative density of their fellow quorum members. Moreover, the release of AHLs operates via a positive feedback mechanism- higher concentrations of AHLs attract more bacteria, resulting in even higher concentrations of AHLs.
Bacteria don’t employ quorum sensing simply because they enjoy each other’s company. In fact, there are almost certainly trade offs associated with group life, including higher levels of predation, lower oxygen availability and buildups of toxic waste products. However, the benefits that can be obtained by living in groups are also significant. Soil bacteria produce extracellular enzymes that release soluble, digestible molecules into their environment. A greater concentration of bacteria results in a greater concentration of free enzymes and thus a more nutrient-rich environment. In the rhizosphere, high concentrations of bacteria can also exert positive feedbacks on rhizosphere priming, the mechanism by which plants release sugars into the soil to nourish the bacterial community.
Though bacterial QS behavior has been well studied for decades, most research has been in the field of disease ecology and few studies have experimentally demonstrated QS to be an important phenomenon in soils. One recent study tackled this problem through a detailed examination of bacterial populations in rhizosphere soil, using controlled pot experiments. The authors found that AHL, the QS signal specific to proteobacteria, was 10 times more concentrated in rhizosphere soils compared to bulk soil. Similarly, bacterial densities in the rhizosphere were about 10 times higher. Furthermore, the researchers discovered a tight correlation between QS and enzyme activity. In particular, enzymes involved in acquiring nitrogen were closely linked to QS expression. Nitrogen is considered the major plant-limiting nutrient in temperate ecosystems, and it is believed that one of the primary motivations for a plant to “prime” the soil around its roots is to access soluble nitrogen from microbial decomposers.
I knew almost nothing about quorum sensing before reading this paper. As always, I found myself overwhelmed by the nuanced mechanisms that bacteria employ in order to explore and shape their environment, coupled with the interactions of plants that can manipulate this complex community for their own nutrition. Quorum sensing could well be an important control in rhizosphere nitrogen availability, and will have to be studied in more detail to fully assess its role in controlling ecosystem productivity.
Reference
DeAngelis, K.M., Lindow, S.E. & Firestone, M.K. Bacterial quorum sensing and nitrogen cycling in rhizosphere soil. FEMS Microbiology Ecology 66, 197-207 (2008).
Protozoa drive growth enhancing hormone release in the rhizosphere: where biochemistry meets ecology
The microbial loop theory: 30 years of cross-Atlantic communication barriers
The more forest ecologists learn about plant nutrients, the more evidence accumulates that plants are not simply passive organisms whose chances of survival are based on environmental factors outside of their control. In acquiring basic nutrients from the soil, one may well imagine that a plant’s success is dependent on chemical properties of the soil alone. By simple “luck of the draw”, plants that seed in nutrient-rich spots will grow faster and larger than plants seeding in nutrient poor regions.
In several of my previous posts, I’ve addressed this issue one way or another, talking about plant-mychorrizhal associations and root-grafting as strategies that allow less fortunately placed plants to acquire sufficient nutrients to survive. I’d like to now address an entirely different theory concerning plant nutrient acquisition, one which, despite thirty years of European research, remains hotly contested and represents one of the major theoretical divides between European and American soil/plant ecologists.
The microbial-loop theory is a paradigm developed several decades ago and has become a cornerstone of European thinking about how plants interact with other soil organisms. In essence a relatively simple idea, the microbial loop would, if proved, require the reevaluation of a huge body of North American literature about plant nutrient acquisition, which generally argues that that basic nutrient demands and stoichiometric constraints- most notably nitrogen limitation in temperate forests and phosphorous limitation in the tropics, exert a fundamental control over forest productivity.
It is well known that plants exert significant control over the processes that occur in the rhizosphere, a narrow zone of soil and pore space that surrounds their roots. Here, plants dump simple sugars such as glucose in order to nourish an active microbial community. They apparently do so because microbes exhibit a diverse array of metabolic capabilities that plants themselves do not have. Microbial processes release essential nutrients, such as nitrogen, from complex organic matter in a plant-soluble form. This much about plant-microbe symbioses- trading carbon for nitrogen or another plant-limiting nutrient- is agreed upon by American and European scientists.
We start entering hot water when we look more closely at the actual microbial players in this game- who are they and what exactly are they doing? “Microbe” is really a very generic term that can refer to pretty much any organism that is invisible to the unaided eye. Within this umbrella grouping, two slightly more specific classess of organisms seem to be important in the rhizosphere: protozoa and bacteria. Bacteria are the tiny prokaryotic organisms that are largely responsible for decomposition and the release of plant-available nutrients. Protozoa, however, are single celled eukaryotes. They are larger, have more complex cellular organization, and importantly, feed on their smaller bacterial neighbors. Any soil sample that contains bacteria almost certainly contains protozoa as well. The relationship between these two groups of microorganisms represents a classic and well-studied predatory-prey model.
So, given that plants are feeding microbes by dumping sugar into the soil, who is the sugar intended for? The bacteria, or the protozoa? The classic paradigm would argue that the bacteria, as the important nutrient-acquiring organisms, are the intended recipients of plant carbon exudates.
But what does this make the protozoa? Are they just thieves, stealing a farmer’s corn that was intended to feed his cattle? Numerous studies have shown that protozoan populations increase dramatically in the presence of plant carbon exudates because they are using the carbon themselves. A high-energy, readily available food source is just as appealing to protozoa as it is to bacteria. Why would plants, that have perfected so many survival strategies over evolutionary time, allow this to happen?
The microbial loop theory argues that it is the protozoa that plants are “cultivating”. Why? Protozoa prey on bacteria, and bacteria, remember, are full of the nutrients that plants need. After eating a bacteria filled meal, a protozoa will likely excrete those same nutrients, making them available for plants. The protozoa are a conduit, passing nutrients to plants that would otherwise be locked up in the bacterial community.
There is mounting evidence from various lines of research in support of the microbial loop theory. Experiments have shown that early in development, plant root architecture is dramatically altered in the presence of protozoa. Increased root branching increases surface area, or “real estate” that protozoa can inhabit. “Tracer” studies, using a labeled isotope of a nutrient, are now providing evidence for a flow of soil nutrients from bacteria to protozoa before becoming plant-available. Finally, molecular studies of bacterial communities reveal an increased abundance of less-palatable bacterial species in the presence of protozoa, and an increased frequency of genes involved with bacterial defense. This genetic evidence underscores the importance of protozoan predation in structuring bacterial communities. Soon, perhaps, nano-cameras will be available to visualize what is actually happening in the rhizosphere between plants, bacteria and protozoa.
The importance of understanding this interaction is not trivial. The means by which plants get their nutrients has ramifications for ecosystem productivity, ecosystem nutrient cycling, and responses to environmental change. Should we progress forward in the field of ecosystem science, a critical reexamination (and open discussion!) of what exactly is going on in the rhizosphere between plants and they critters they cultivate is necessary.
A detailed review of microbial loop theory and a paper that addresses some of the important counter-arguments:
1. Bonkowski, M. Protozoa and plant growth: the microbial loop in soil revisited. NEW PHYTOLOGIST 162, 617-631 (2004).
2. Ekelund, F., Saj, S., Vestergard, M., Bertaux, J. & Mikola, J. The “soil microbial loop” is not always needed to explain protozoan stimulation of plants. SOIL BIOLOGY & BIOCHEMISTRY 41, 2336-2342 (2009).
deep sea carbon cycling: more dynamic than we thought?
For years, scientists have speculated that deep sea carbon may have played an essential role in past climate change episodes. Specifically, it has been suggested that the C bound in seafloor sediment has undergone thermodynamic alterations in the past to due upwellings of molten magma from the mantle. Magma may have triggered the release of CO2 and methane into the upper ocean and eventually the atmosphere. Evidence suggests that the Paleocene-Eocene thermal maximum, which lasted approximately 100,000 years, may have been triggered in part by the release of greenhouse gases from the seaflooor.
Despite these speculations about deep sea carbon influencing past climate, little research has been done on the role of seafloor carbon in the present day C cycle. In some ways, this is surprising given the enormous amount of attention being payed to global C budgets and possible means of C sequestration. It is generally assumed that the deep sea represents a huge “carbon sink”, to which organic C from the upper ocean enters and does not emerge again for thousands to millions of years. This would suggest that whatever carbon-cycling processes are occuring at the seafloor are not powerful enough to cause a net carbon release.
Recent research published in Nature Geoscience suggests otherwise. Several case studies have demonstrated dynamic processes occurring on the ocean floor can in fact lead to a net release of greenhouse gases. Spreading seafloor centers- regions where oceanic plates pull apart- are a site of magma activity and hydrothermal venting. Hydrothermal vents release a variety of hot, mineral-rich fluids that can support a diverse microbial and invertebrate community. At one such spreading center in the Gulf of California, magma is intruding into thick organic basin sediments. These sediments have long been thought to sequester C, however, it now appears tht their heating is causing the release of methane into the upper ocean.
In the Northeast Pacific, another intriguing deep ocean C cycling system has been discovered. Here, microbes are converting ancient inorganic C into dissolved organic C, which is subsequently released to the overlying ocean. This discovery contradicts the general belief that ancient deep-sea C is highly stable and not accessible to microbes.
Other distinct seafloor C sources are rapidly emerging around the world, as improved technology and a heightened interest in seafloor processes are accelerate the pace of discovery. However, the contribution of such “point sources” to global C budgets is still highly uncertain and far more research is needed to come up with even a rough estimate of global deep sea C sources. Nonetheless, it would seem that we can no longer consider the deep ocean a black box of C sequestration, and that we should think carefully about the ramifications of introducing more carbon- either accidentally through the introduction of dissolved greenhouse gases to the ocean, or intentionally as part of a climate change mitigation strategy- to a system that is clearly more dynamic than we once thought.,
Reference : “Deep Sea Discoveries.” 2011. Nature Geoscience: Letters. Volume 4, Page 1.
microbiologists watch evolution in action
rapid advances in molecular genetics are now allowing scientists to watch, and even manipulate, evolution. this may seem hard to reconcile with the idea that evolution is a very gradual, long-term process, whose effects are seen only on timescales of thousands or millions of years. the misconception here is that time governs the rate of evolution. time in the abstract is relentless and constant, moving forward endlessly. this conception of time does not take the broad range of life strategies that evolution has produced into account.
in fact it is generation time that governs rates of evolution. human beings, who tend to have several children over the course of a multi-decadal life, have relatively slow generation times. it can take millions of years for noticeable evolutionary shifts to occur in a population that grows and reproduces slowly, simply because the genetic mutations that lead to evolution are very rare, and advantageous mutations take a long time to become established in a population.
it has long been known that microbial evolution occurs rapidly- noticeable genetic shifts can be observed in a manner of days or weeks. the evolution of pathogen resistance to pesticides or medicine occurs through natural selection for a rare genetic mutation that allows survival. because microbial populations often grow exponentially and generation time can be as short as twenty minutes, rare genetic mutations can sweep through a population and become ubiquitous rapidly. this is evolution in action!
historically, experiments in microbial genetics have focused on determining the function of an existing gene. this is generally accomplished by creating a strain with a defective, mutant version of the gene of interest, and observing how its function differs from the normal gene. studying defective mutants, however, does not provide insight into how gene pools can be improved.
now scientists are growing microbial cultures whose entire genetic makeup is known, and performing experiments that test evolutionary theory. any number of questions are being asked- how do the bugs evolve in response to an environmental change? a new food source? the introduction of a genetically different strain? for example, if a microbial population that requires oxygen to breathe is suddenly placed in a low oxygen environment, will genetic shifts occur that allow the microbes to use oxygen more efficiently? this certainly seems to be the case with humans- human populations that have existed for centuries at high altitudes, where oxygen is scarce, exhibit slight alterations in genes that encode hemoglobin, the protein that binds oxygen and transports it throughout the body. controlled evolution experiments allow replication, which means that scientists can now ask how frequently a positive evolutionary outcome, such as increased oxygen efficiency, occurs.
though such experiments may only provide a simplistic illustration of evolution, the mechanisms leading to genetic changes in microbial populations are remarkably similar to the basic mechanisms governing genetic change in higher organisms, including humans. insights developed from these experiments may be a first step towards unraveling the complex chain of events that has created the extraordinary diversity of adaptive traits across all types of life.